New Generation Aqueous Ink Base Material for Packaging Film and Paper (2)
MFFT measurement
MFFT is defined as the lowest drying temperature at which latex can form a uniform, transparent, and crack-free coating. For the measurement, a wet film of 60 μm in thickness was first prepared with a coating bar MFFT Bar SS-3000 manufactured by Sheen Instruments. After the film was completely dried after 30-60 min, the film was visually observed to be coated by milky white or a crack. Membrane becomes the transition point (temperature) of the transparent attached film and MFFT is measured. The visual measurement temperature tolerance is ± 0.5 °C.
AFM measurement
AFM measurements were performed using an open cell NanoScope III digital instrument.
Dryness
The dryness was measured on a 100 μm thick latex wet film coated on a 100 cm 2 aluminum plate. The above aluminum plate was placed under standard environmental conditions, and the plate mass was recorded once every 20 seconds until it was kept constant, and then left at 150C overnight to completely remove the water.
Reversibility test
A 12 μm emulsion (a few drops of pigment slurry was added) was prepared on a paper substrate and placed on a wet film at room temperature 22 ± 2 °C and relative humidity of 45% for 1 h, and then dried again on the dried film. The same latex was used to remove the latex droplets with a wet fabric after some time. The dripping emulsion polymer completely dissolves on the colored polymer film, indicating reversibility or redispersibility.
Anti-back tack determination
A 12 μm latex wet film was prepared on a paper substrate and dried at 80°C for 10 s, then the two coated test pieces were placed face-to-face with a coating surface, and a load of 1 kg/cm 2 was added at 52°C. After 16 h at °C, the load was removed and allowed to cool to room temperature (22 ± 2 °C). If the two coatings could be separated and the membrane was not damaged, it showed good resistance to re-tack and rated at grade 5. If the two layers are completely bonded together, the anti-back tackiness is considered to be poor and rated as 0.
Rheological measurement
Rheology is studied by measuring the relationship between the low shear viscosity of a polymer and the fill ratio or solids content. The Krieger-Dougherty equation is used to represent the maximum filling ratio φ m of the system.
η r = η / η s = [1- φ / φ m ]-[ η ] φ m Equation 1
Here η r denotes the relative viscosity, η is the viscosity of the dispersion, η s the viscosity of the suspension, φ is the volume fraction, φ m is the volume fraction at maximum filling, and [η ] is the intrinsic viscosity (2.5 for rigid spheres). Rheology was measured with a Bohlin CS rheometer.
Results and discussion
Rheology Measurement of the flow profile of a mixture of polymers/oligomers containing both size and morphology (both softer) at oligomer concentrations of 10% and 30%. In past work, the viscosity (η) of the dispersion at each low shear rate for each solid content was determined and the relative viscosity η r was calculated. The relationship between the calculated relative viscosity and volume fraction of each solid content is shown in Figure 3.
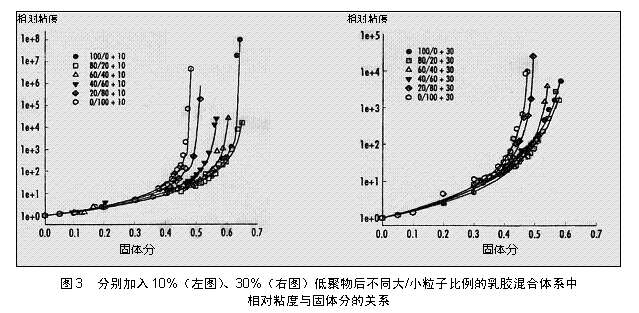
The data shows that there is a typical dispersion characteristic, not a polymer solution. At low concentrations the viscosity changes little with solids, whereas at high solids the relative viscosity rises rapidly. This data conforms to the Krieger-Dougherty equation.
The above data shows that the maximum filling fraction φ m can be used to characterize the dispersion and φ m is the volume fraction value when the viscosity reaches infinity when the particles are closely packed. For rigid single-form spheres, regardless of particle size φ m =0.64, and in both size and form mixing systems, φ m values ​​can certainly be higher by filling more effectively, as in the previous article, pure small particles or large The φ m values ​​of the particle system are 0.4 and 0.54, respectively.
Figure 3 shows the change of the ratio of two-component latexes, the ratio of solid content to relative viscosity, after adding 10% (left) or 30% (right) oligomers, respectively.
These values ​​are all lower than 0.64, because the actual dispersion is not a rigid sphere, which is due to the repulsion between particles caused by the double layer of the particles. Therefore, the particle volume fraction seems to be higher in hydrodynamics than the value measured by gravimetric method. The smaller the particles, the more obvious this is, when the electric double layer can increase a considerable part of the particle volume fraction.

Figure 4 shows the relationship between φ m and the size of the particles in the two morphological polymer/oligomer systems. In a system containing two types of morphologies containing a reversible oligomeric phase, the peak of φ m appeared when the ratio of the size particles was 80/20, and the results when the oligomer was not included were similar.
However, the maximum φ m value decreases from 0.62 without oligomers to 0.58 with 0.5% and 10% with 30% oligomers, respectively. It is expected that the maximum solids content of fully dissolved, non-interacting polymers may increase as the concentration of oligomers increases because high dispersion concentrations can effectively be diluted with polymer solutions. This experiment has noticed this effect, for example, the introduction of low molecular weight polyethylene glycol. However, this oligomer can be adsorbed on the particle surface to increase the effective (hydrodynamic) volume fraction. For this type of oligomers, instead, the maximum filling ratio will be reduced. Obviously, compared to the case of using small particle dispersions, the φ m of the mixture system of both size and morphology is greatly improved.
In summary, rheological data indicate that the maximum solids content can be achieved with a resin system containing two types of morphological particles added with an alkali-soluble oligomer. Note that all of the above maximum fill ratios refer to volume fractions rather than solids. For the 0, 10%, and 30% oligomers, respectively, the corresponding maximum solid content was 0.70, 0.66, and 0.62, respectively.
Dryness
For the study of dryness, a mixture of large and small particles was prepared and 0, 10% and 30% oligomers were added. The drying time measured for all samples, ie the time when the maximum solid content was reached, is shown in Table 3. At the same time, the drying time of a single oligomer (20% solids) was measured, soft oligomer was about 66 min, and hard oligomer was 58 min. These results indicate that the addition of oligomers generally increases the drying time. This can be explained by the fact that the oligomers have a high hydrophilicity due to the high acid content and that they retain more moisture in the film, which reduces the Dryness. The addition of small amounts of oligomers has greatly affected the drying time of the entire system, and continued increase in the amount of oligomers does not significantly increase the drying time.
As described in the previous literature, except in systems with high Tg-containing oligomers, at the same solids fraction, the drying speed of the mixed system of the two particle forms was slightly slower than that of the single-form system. To determine the drying rate, a dry curve was obtained by plotting the time vs. solids relationship. In Figure 5, three different bases for aqueous inks are provided: oligomers, oligomers/monomorphic PSD polymers (0/100, mass ratio, large/small) and oligomers/two forms of PSD Polymer (80/20, mass ratio, large/small) dryness curves.
These PSD polymers containing two sizes of morphology can produce higher solids content (see rheology section), which can make the drying time shorter than traditional ink base materials. The use of one-step preparation of oligomer/polymer samples of two particle size distributions (using surfactant penetration) can result in higher solids content, which further accelerates drying.
A Great Addition to Outdoor Garden Decoration
The adorable solar statue is weather-proof, rust-resistant, and durable for years of quality use. With its artistic look and intricate details, the outdoor sculpture adds a delicate touch of illuminating beauty at night.
Please Note:
1. Make sure the button is set to the "ON" position and keep charging for 10 hours under the sun at the first using.
2. It may take longer time to full charge if there is no strong light or in Winter. Please do not let it covered by the leaves, flowers or trees, or it can`t absorb solar energy or will light up at daytime.
Effective Solar Panel
During the days the solar panel collects sunlight and converts it into electrical power. Automatically turn on at night or in dark environment, auto turn off at dawn.
FAQHow to know that the button is at "ON" position?
Cover the solar panel with your hand and if the light is on, then the button is at "ON" position.
Solar Statues,Plant and Lawn Stake, Garden Stake Ornaments, Garden Stakes Decor, Decorative Metal Stakes
HISMOK(SHENZHEN)TECHNOLOGY CO.,LTD , https://www.will-trade.com