Specific heat capacity concept and calculation
1. Definition of specific heat capacity:
A substance of unit mass, the temperature rises (or decreases). The amount of heat absorbed (or released) at 1 °C is called the specific heat capacity of the substance. It is indicated by the symbol c.
2. Specific heat capacity unit:
Coke / (kg · Celsius), the symbol is J / (kg · ° C), read as coke per kilogram Celsius, it means the physical meaning is: a certain mass per unit mass temperature rises (or decreases) l ° C, absorb How much heat is (or released)?
3. Calculation of specific heat capacity
There is an object of mass m, and when the heat ΔQ is absorbed (or released) in a certain process, the temperature rises (or decreases) ΔT, then ΔQ/ΔT is called the heat capacity of the object in the process (referred to as heat capacity). Expressed by C, ie C = ΔQ / ΔT. Dividing the heat capacity by the mass, that is, the specific heat capacity c=C/m=ΔQ/mΔT. For the heat capacity and specific heat capacity of the micro process, there are C=dQ/dT and c=1/m*dQ/dT, respectively. Therefore, in the finite process in which the temperature of the object changes from T1 to T2, the heat absorbed (or released) Q = ∫ (T2, T1) CdT = m ∫ (T2, T1) cdT.
In general, the heat capacity and the specific heat capacity are both functions of temperature, but when the temperature change range is not too large, it can be approximated as a constant. Then there is Q = C(T2-T1) = mc(T2-T1). If the temperature change amount ΔT = T2 - T1, then Q = cm ΔT. This is the basic formula for calculating calories in secondary schools using specific heat capacity.
In English, the specific heat capacity is called: Specific Heat Capacity (SHC).
The formula for calculating thermal energy with specific heat capacity is: energy = mass × specific heat × temperature change
Can be abbreviated as: Energy=SHC×Mass×Temp Ch, 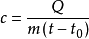
The heat calculation formula related to specific heat: Q=cmΔT, ie Q absorption (release)=cm (T-T-end) where c is specific heat, m is mass, and Q is energy heat. When the heat is absorbed, it is Q=cmΔT liter (subtracting the initial temperature of the object with the actual elevated temperature), and Q=cmΔT is decreased when the heat is released (the temperature after the actual initial temperature is reduced). Or Q=cmΔT=cm (T-T-T), when Q>0, it is endothermic, and when Q<0, it is exothermic.
The formula for calculating the specific heat capacity is generally 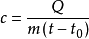 .
.
Q suction = cm (t-to) Q release = cm (to-t)
c represents the specific heat capacity.
m represents the mass of the object
To indicates the initial temperature of the object
t indicates the end temperature of the object
(â–³t: object change temperature, ie t-t0)
This is used to calculate the formula when the temperature of the object rises. If the object is lowered, the initial temperature of the object is subtracted from the end temperature. which is.
The formula for calculating specific heat capacity is also written 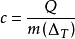
Rattan metal chair
STARWAY INTERNATIONAL HOME-LIVING CO., LTD , https://www.starwayfurniture.com