Zong Weijian: A new generation of miniature two-photon fluorescence microscope
From the cult of the primitive tribes of the Stone Age to the worship of the soul, to the philosopher's trace of the brain consciousness in the late Middle Ages, to the modern eradication planners to find the orderly brain function partition, and then in the early 20th century, Santiago Cajal got the human A clear picture of the cerebral cortical neurons, until now neuroscientists have studied the structure and function of the brain at subcellular, molecular, and genetic levels through electrophysiology, electron microscopy, and optical microscopy. Neurosciences This ancient discipline, to this day, remains one of the most active and active scientific research fields in the world.
The most important factor limiting scientists to understand and explore the brain is technology. Every major breakthrough in the field of neurology is based on the revolution and leap of technology. The invention of the Golgi-staining and Nice-staining techniques at the end of the 19th century led to the complete and clear presentation of the structure of a single neuron, summed up by the father of modern neurology, Santiago Ramon y Cajal (1852-1934). The creation of neuron theory is still the foundation of modern neuroscience. Development of non-invasive imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), transcranial Doppler (TCD), single photon emission computed tomography (SPECT), and positron emission tomography (PET), It has enabled humans to continuously improve their understanding of the overall horizontal structure and function of the brain, and provides a useful reference tool for the treatment of brain trauma and disease. In the field of experimental neuroscience, model animals are used as research objects, which avoids the limitation of ethics such as invasiveness and transformation as a research object, and makes more technical means to show their talents. Including electrophysiology, electroencephalography (EEG), multi-electrode recording (MER), patch clamp and other techniques for the invention and effective use of scientists to enable sub-micron spatial scales (single synaptic connections) ), sub-millisecond time scale (single nerve impulse potential) to study the function of neurons. The most exciting thing is that the booming optical microscopy imaging technology in recent years has brought a lot of unprecedented ideas and results to experimental neuroscience. In 2008, Qian Yongjian and others won the Nobel Prize in Chemistry for their discovery and use of fluorescent protein (GFP, green fluorescent protein), which is a great affirmation and promotion of fluorescence imaging technology. Optical imaging itself has high-resolution, high-throughput (high-speed), non-invasive, non-toxic characteristics, combined with the positioning and expression technology of fluorescent proteins and fluorescent dyes in cells, making scientists specific It distinguishes different structures and components in living organisms and even inside cells, and can dynamically observe its function in a state in which living bodies and cells are still active (in vivo state). This makes fluorescence imaging technology an irreplaceable one of the most important technical tools of biologists today. With the emergence of various new microscopic techniques in recent years, confocal microscopy, coherent Raman imaging (CARS), super-resolution microscopy, light microscopy (lightsheet microscopy), etc. make the resolution, speed, imaging depth, etc. of the fluorescence microscope further improved.
For the application of fluorescence imaging technology in neuroscience, two-photon microscopy (TPM) 1 is indispensable. At present, most cell biology and physiology studies are mainly studied in cell systems cultured in vitro. However, unlike cell biology research, the holistic and in situ nature of brain function studies is even more critical: only studying isolated neurons cannot explain the function and regularity of the nervous system. In other words, neurons must be required to be in their normal living brain environment in order to function properly. However, the brain is a highly complex organ. Even the cerebral cortex of mice has a thickness of nearly 1 mm. The deep brain regions such as hippocampus and thalamus are as deep as 3-5 mm2, and are opaque, filled with hundreds of millions of neuronal cell bodies and synapses. It is rich in blood vessels, mucous membranes (meninges), and the outermost layer is covered with thick skull and scalp. Using a conventional fluorescence microscope, including a confocal microscope, the observed signal is scattered and absorbed by the sample tissue, and it is impossible to penetrate such deep tissue for imaging. The invention of two-photon microscopy has brought hope to such research. The unique nonlinear optical properties of the two-photon microscope, combined with the operating wavelength in the infrared region, greatly increase the penetration depth in the living tissue, making the two-photon microscope the most neuron imaging for living scientists. The ideal tool. The action potential itself is difficult to capture by optical signals, but the depolarization produced by the action potential causes changes in the concentration of Ca2+ in the neurons (calcium influx). Scientists have developed fluorescent probes with various concentrations of Ca ions to reflect neural activity through changes in the fluorescence signal caused by changes in the concentration of calcium ions. Thus, the two-photon microscope combined with the in vivo neuron Ca ion concentration indicator labeling technique collides with a dazzling spark: enabling one to study neuronal activity in the animal's brain when in a physiological state4.
The most important function of the brain is to regulate the behavior of the organism, and conversely, the behavior that best reflects the working state of the brain is also the behavior of the organism. Therefore, in order to understand the brain, the researcher not only requires high-resolution observation of neurons in the body state, but also hopes that the organism can perform normal behavior during the observed phase. Therefore, while imaging technology continues to improve the performance of resolution and speed, scientists are also actively improving and imaging these imaging techniques to minimize the behavior of the observed objects when imaging. Get the data closest to the physiological state. However, there are always many technical bottlenecks in this goal: Take two-photon calcium imaging of rodent (rat or mouse) neurons as an example. In the early years, the shaking of the animal's body motion was severe, and at the time the imaging speed of the two-photon microscope was very low, so scientists could only image the animals fixed in the head under anesthesia. Later, with the increase of imaging speed and the great improvement of craniotomy, scientists can observe the nerve activity of animals in awake state (still need to fix the head). In recent years, with the rapid advancement of genetic modification technology, endogenous expression of "genetically encoded calcium indicator (GECI)" in neurons has become a neuronal calcium imaging through viral transfection and transgenic technology. Megatrend 4. This method of producing calcium indicators by the neurons themselves has a huge advantage over the previous calcium dye technology: the signal-to-noise ratio is increased by an order of magnitude; the neuron specificity is good, and different neuron types can be distinguished; Continuous expression in brain neurons for several months (viral transfection) or even entire life course (transgenic animals). So, about 10 years ago, scientists began to use long-term observation and tracking of neuronal activity and structural changes using two-photon imaging combined with GECI technology, thus gaining a deeper understanding of memory formation, neuronal disease and other issues. . Among them, the best performance now, the most widely used GECI is the green fluorescent calmodulin Gcamp family 4. Currently improved to the sixth generation, Gcamp6f, Gcamp6f has become one of the most popular indicators in neuroimaging. At present, the most popular method for scientists to image brain activity during the behavior of small animals is to combine virtual reality with two-photon imaging. When the animal's head is fixed, the image is made in front of the eye, and the animal is considered to be at home. In the "real" environment 5. Simulate its real activity by moving the limbs of the mouse on a treadmill or mouse ball. In order to achieve the role of studying neurons in animal behavior (Figure 1).
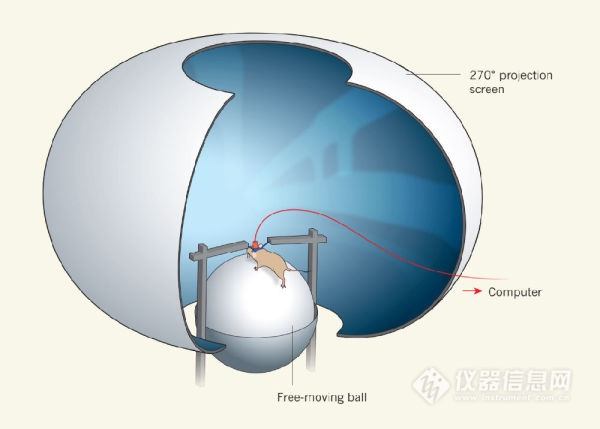
Figure 1 Two-photon imaging combined with virtual reality scenes to study animals with fixed heads and physical activity. Picture from 5
However, this method of virtual reality plus head fixed imaging has been questioned by many scientists. It is believed that animals with fixed heads have been under physical constraints and emotional stress during the experiment, so it is impossible to prove that the response of neurons to the outside world is equivalent under virtual reality and free exploration. More importantly, many social behaviors, such as parent-child care, mating, and fighting, cannot be studied with head-fixed experiments. How to directly image the neurons while the animals are free to move is a neuroscientist who has not yet been able to solve the ultimate appeal.
An ideal solution is to develop a micro-fluorescence microscope that is directly attached to a free-moving animal, allowing the animal to "run with a microscope"6. This kind of attempt started about 20 years ago. Initially, scientists simply inserted one or several fibers into the mouse's head for laser introduction and fluorescence signal acquisition. However, this method only records the sum of the signals in a certain area, does not have spatial resolution, and is not really imaging. In the last decade or so, due to the development of optics, electronics, and materials technology, people began to try to develop microscopic microscopes in the true sense. Among them, the miniature wide-field microscope, due to its relatively simple principle and structure, is currently the main micro-microscope technology. For example, a microscope developed by Ghosh and colleagues, by integrating a small LED light source, a miniature CCD, and a self-focusing lens into a frame of less than 25px3, developed a miniature wide-field microscope with a weight of 1.9g. This technique was used to study the interaction between memory cells and instinct in the hippocampus of the brain. However, the wide field imaging method cannot achieve cell resolution because it does not filter the background signal of the defocused area well and is sensitive to light scattering. It is more difficult to observe finer structures such as dendrites, axons, and dendritic spines. So it has been difficult to achieve satisfaction with neuroscientists.
Thus, from about 15 years ago, some research groups in the world that researched and developed two-photon imaging technology began to experiment with two-photon microscopy, a technology that has been widely used in the field of neuroimaging. However, only a handful of research groups have reported their progress in the development of miniature two-photon microscopy: In 2001, work by Denk et al. was considered the first step in the development of a miniature two-photon microscope. However, it is still too "huge" (7.5 cm long and weighs 25 grams) and the imaging speed is very slow (2 Hz at 2 Hz 128x128 and 0.5 Hz at 512x512, as shown in Figure 2a). Later, other research groups have reported different micro-two-photon systems. The Helmchen team reported their miniature dual-optical subsystem in 2008, weighing only 0.9 grams. It achieves an imaging speed of 8 fps at 512 x 512 and demonstrates the in vivo calcium imaging signal achieved with the system. However, from the effect of the display, its spatial resolution is extremely low, and it does not achieve true free motion imaging (Figure 2b). The Mark Schnitzler team also published their miniature dual-photonic subsystem 10 in 2009. Their system first used a microelectromechanical scanning mirror (MEMS) for scanning and integrated the Z focus module into the probe (Figure 2c). However, the scanning frequency is still very low (400x135 is about 4Hz); the spatial resolution is far below the requirement (1.29 μm laterally, 10.3 μm axial). These aspects limit its use in imaging neuronal cell nuclear subcellular levels. The Kerr team demonstrated their system11 in 2009. Compared to the previous miniature two-photon microscope, the system has a higher spatial resolution due to the use of a miniature objective lens (NA up to 0.9). However, the weight of this probe is also increased (5.5g). In addition, since it still uses a vibrating fiber for scanning, its imaging speed is still relatively slow. (100.9 Hz for 64x64, 1.25 Hz for theoretical 512x512) (Figure 2d). In addition, there is a problem that has not been solved by all previous micro-two-photon systems. Because micro-two-photon microscopes generally need to use fiber optics to introduce femtosecond laser into the probe, and because of the existence of a series of limitations such as dispersion, cut-off mode, and conduction bandwidth, a certain fiber generally only allows a certain bandwidth (generally Tens of nanometers) and light propagation at a specific central wavelength. It is then necessary to select the fiber at the laser wavelength required to combine the fluorescent indicator used in the fabrication of the microscopy. However, the currently commercialized hollow-core photonic crystal fiber (HC-PCF) type that can be used for femtosecond optical transmission is very limited. For example, NKT, the world's largest producer of photonic crystal fibers, offers only HC-PCF with center wavelengths of 800 nm, 1030 nm, 1300 nm and 1550 nm. All existing miniature two-photon microscopy imaging systems are developed based on the center wavelengths defined by these fibers. Unfortunately, the most widely used GcamP indicator mentioned above in this article requires a 920 nm laser for excitation. So all previous miniature two-photons could not effectively image Gcamp. This limits the development of miniature two-photon microscopy.
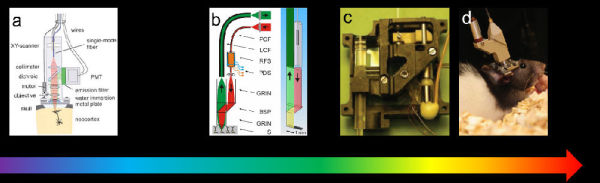
Figure 2 shows some typical work in the history of micro-two-photon development. a, b, c, and d are selected from references 8, 9, 10, and 11, respectively.
The reason why these early miniaturized two-photon microscopes could not be truly used and promoted is that the fabrication of practical miniature two-photon microscopes is much more complicated and difficult than the development of single-photon micro-microscopes. Micro-two-photon microscopy needs to address several key technical challenges:
1 How to effectively introduce a femtosecond laser into a micro microscope;
2 How to perform scanning/image reconstruction in a micro microscope;
3 How to perform high-quality laser convergence in a micro microscope to efficiently excite two-photon signals.
4 How to effectively collect fluorescent signals;
5 How to make the whole system stable even when the animals are strenuous
6 Under the first five conditions, whether the weight is light enough to affect the animal's activities as little as possible;
The research group of the author is composed of the Interdisciplinary Team of the Institute of Molecular Medicine, the School of Information Science and Technology, the Center for Dynamic Imaging, the School of Life Sciences, and the School of Engineering of the People's Liberation Army. Under the leadership of Academician Cheng Heping, under the support of the National Natural Science Foundation of China, the National Science Research Instrument Development Project, "Super High-temporal Time-Resolved Miniaturized Two-photon In-vivo Microscopic Imaging System", after more than three years of concerted efforts, success A new generation of high-speed, high-resolution miniature two-photon fluorescence microscope was developed and named FHIRM-TPM. The original paper was published online May 29 in Nature Methods (IF 25.3)12. In this work, we solved the problems in the development of the earlier miniaturized two-photon microscopy mentioned above, and obtained clear and stable images of brain neurons and synapses in mice during free behavior.
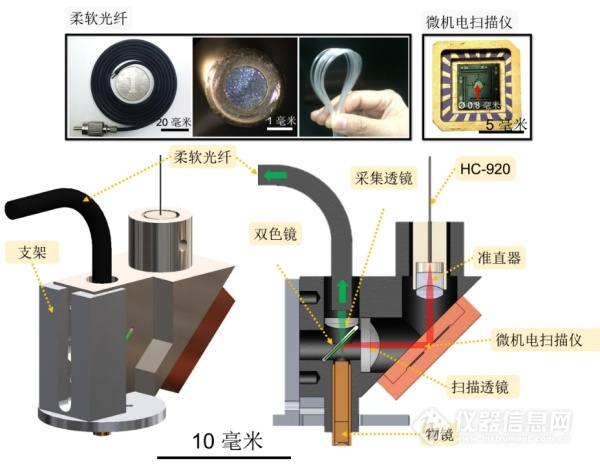
Figure 3 FIRM-TPM schematic from 12
The new generation of miniature two-photon fluorescence microscope is small in size and weighs only 2.2 grams. It is suitable for wearing on the head of small animals. It records the dynamic signals of dozens of neurons and thousands of synapses in real time through the cranial window. In large animals, long-time observations of multiple probes and different brain regions of multiple cranial windows are also expected. Compared with single-photon excitation, two-photon excitation has the advantages of good optical tomography and deeper biological tissue penetration, so the imaging quality is far superior to the miniaturized wide-field microscope developed by the core team of the US brain science program. . Its horizontal resolution reaches 0.65μm, which is comparable to the commercial large-scale bench-top two-photon fluorescence microscope. The dual-axis symmetric high-speed MEMS rotating mirror scanning technology has an imaging frame rate of 40Hz (256*256 pixels). Area random scan and line scan capability of 10,000 lines per second. Most importantly, the FHIRM-TPM overcomes two obstacles to the previous limited dual photon microscope application. First, our custom-designed HC-PCF provides distortion-free transmission of 920 nm femtosecond laser pulses, an improvement that makes it possible to efficiently stimulate common fluorescent indicators such as Thy1-GFP and GCaMP-6f. Second, due to the high spatial resolution and layer-cutting capabilities of the two-photon point scanning microscope, miniature two-photon microscopes mounted on animal heads are very susceptible to motion artifacts. To solve this problem, we have fully optimized the entire system: (a) using a soft new fiber bundle SFB to minimize the torque and pulling force caused by animal motion, without reducing photon collection efficiency; A separate rotatable connector to connect the fibers and wires on the optical probe to minimize distortion and entanglement of the animal during free exploration; (c) use high speed imaging to reduce motion-induced intra-frame blur. In addition, we pre-trained the animal to adapt to the micro-microscope mounted on its skull and added 1.5% low-melting agarose to fill the objective lens and brain tissue before the experiment. These measures significantly reduced the probe between the brain and the brain. The relative motion, which in turn improves the short-term and long-term stability of the experiment, enables high-resolution imaging in animals performing behavioral experiments involving large amounts of body and head movements.
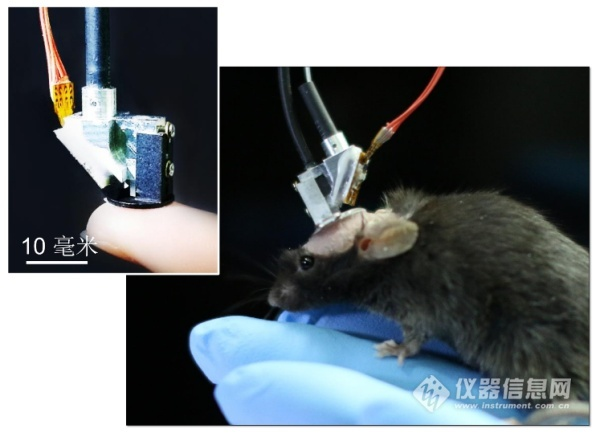
Figure 4 FIRM-TPM physical map from 12
Dendritic spine activity is the basic event of neuronal information processing. Studies on head-fixed animals using bench-top two-photon microscopy have shown that different dendritic spines of individual nerve cells can be stimulated by different directions of visual stimulation or sound of different intensity frequencies. activation. The FHIRM-TPM achieves the same resolution and optical layering capabilities as traditional large benchtop two-photon microscopes. Compared to miniature wide field microscopes, FIRM-TPM's high spatial resolution, inherent optical slicing ability and tissue penetration and considerable mechanical stability are extremely advantageous. So while hundreds of neurons can be obtained at the cellular level by micro-wide field microscopy, our FHIRM-TPM undoubtedly provides a more powerful tool for more basic neurocoding units in free-living animals. - The temporal and spatial characteristics of dendritic spines are observed. It is able to observe the activity of neuronal cell bodies, dendrites and dendritic spines in the brain for a long time during behavioral tests (such as tail-hanging, jumping, and social behavior) in mice. The demonstration of these features fully demonstrates the good performance and stability of FHIRM-TPM. In the future, the combination of optogenetics technology is expected to accurately manipulate the activities of neurons and brain circuits while imaging structurally and functionally. The micro-two-photon fluorescence microscope has stable performance and can be used for long-term observation of synapses under natural behaviors such as animal foraging, jumping, fighting, playing, and sleeping, or before, during, and after learning. Multi-scale, multi-level dynamic changes such as neurons, neural networks, and remotely connected brain regions.
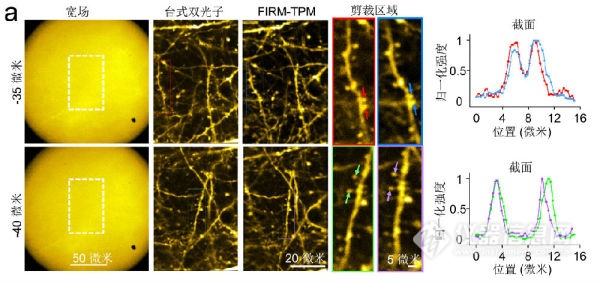
Figure 5 Comparison of imaging quality of three modes in structural imaging, from 12
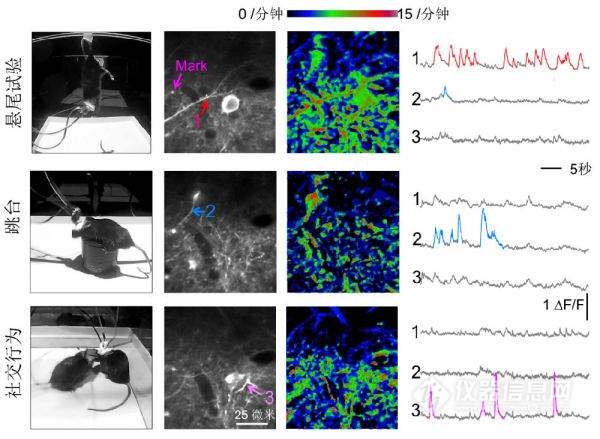
Figure 6 FHIRM-TPM images mouse cerebral cortical neuron activity in three different behavioral paradigms, from 12
Since the first prototype of a miniature two-photon microscope was published by Denk in 2001, the development of miniature two-photon microscopy has been going on for 15 years. After 15 years of development, the miniature two-photon microscope from the very beginning of the 25-gram bulky body can only be tested in separate tissues. 8 to now weighs only a few ounces and can be used for free-moving mice. Neurons perform imaging at the level of dendritic spines, and it can be said that some progress has been made. However, while seeing the achievements in this field, it should also be noted that, so far, miniature two-photon microscopes have not been widely recognized and applied by biologists like confocal microscopes or fluorescent microscopes. The latter (light film microscope) has a shorter development time (a document from Science in 2008 is generally considered to be the beginning of a modern fluorescent microscope microscope 13). The reason, in addition to the limitations of the technology itself, the atmosphere and investment in the entire research field is also an important factor.
Throughout the development road of micro-two-photon microscopy in the past 15 years, there are some people who have developed the territory; those who have reformed and innovated have it; others who have found it; those who fish in the water and the deer as horses also have it. Regrettably, however, there are few people who are willing to be open-minded and full of input; there are few who have the will and ability to establish a paradigm for the field of this research. China, in the recent past, is basically a complete blank in this field. Not to mention what leads the world.
However, it is very exciting that the National Science and Technology Commission's national major scientific research equipment development project officially launched the "ultra-high time-space-resolved miniature two-photon in-vivo microscopic imaging system" in 2014. With 5 years and 7 million yuan of research funding, this research and development of "the world is not very good, China has basically no one done" technology. Such a strong investment has undoubtedly injected fresh blood and full motivation into this field. I was also fortunate to have participated in the project throughout the five-year period. From 2012, I came to the joint research team of the project leader, Dr. Cheng Heping and Chen Liangyi, and I have witnessed the whole process of the project from scratch to maturity. Now, we have a preliminary result, not only let us a team that is completely established by Chinese national scientists in the world's leading position, but also promote this field forward, I feel very much Excited and proud.
The results were reported at the end of 2016 at the American Neuroscience Annual Meeting and the May 2017 Cold Spring Harbor Asian Brain Science Conference. They were highly praised by domestic and foreign neuroscientists including many Nobel Prize winners. Professor Alcino J Silva, president of the Asian Brain Science Conference at Cold Spring Harbor and the famous American neuroscientist UCLA, wrote in the comments: "From any standard, this microscope represents a major technological invention, and it will definitely Changing the way we observe cells and subcellular structures in free-living animals. It opens the door to even beyond neuronal and dendritic imaging. Systematic neurobiology is entering a new era, Identifying the complex biological events of cells and subcellular structures for imaging observations, thus gaining a deeper understanding of the core engineering principles of evolutionary brain loops to achieve complex behaviors. Undoubtedly, this extraordinary invention allows us to A goal has taken a step forward."
Boat Tray,Paper Boat Tray,Disposable Boat Tray,Takeaway Food Container
JIANGYIN BIOPACKAGE CO.,LTD , https://www.chinapulppackage.com